Nuvaxovid
Nuvaxovid contains a version of a protein found on the. Beslutet är temporärt och gäller från.
Trois Questions Sur Le Nuvaxovid Le Vaccin Contre Le Covid De Novavax Autorise En France
1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit.

. As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus. The first batch of Nuvaxovid is expected to arrive in. Novavax is a protein-based vaccine.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. This vaccine called Nuvaxovid produced by the company Novavax is a so-called subunit vaccine and differs from the previously approved vaccines. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Data från Australien pekar mot en ökad.
2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. Active immunisation to prevent coronavirus disease 2019 COVID. What are subunit vaccines.
1 day ago3rd November 2022 0355 GMT11. Rokotteesta ei myöskään ole haittaa vaikka. This type of vaccine contains part of the coronavirus spike protein.
As at 31 May four cases of adverse reactions were reported out of the 2792 doses of Nuvaxovid administered here at a 014 per cent incidence rate the report published on. The addition of the saponin-based Matrix-M adjuvant. Nuvaxovid-rokote sopii lähes kaikille aikuisille.
Clinical trials showed that beginning 1 week after the second dose Novavax Nuvaxovid COVID-19 vaccine was. Nuvaxovid contains a version of a protein found on the. Nuvaxovid SARS-CoV-2 rS with matrix M adjuvant NVX-CoV2373 was approved for the following therapeutic use.
The new vaccine was developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI and is the originator product for the Covovax TM vaccine. This will enable us to start offering the Nuvaxovid. The vaccine is safe and effective for all individuals aged 18 and above.
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Det eftersom att data från Australien gett signaler. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach.
Your immune system cells recognise the spike protein as a threat. 1 day agoDet proteinbaserade covid-19-vaccinet Nuvaxovid ska inte ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista.
Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. Company Novavax should not be given to individuals. In line with the WHO Prioritization Roadmap and the WHO Values Framework older adults health workers.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Nuvaxovid is authorised in Northern Ireland under the CMA granted by the European Medicines Agency on 20 December 2021. This CMA has similar requirements to that.
Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. 90 effective in protecting trial participants aged 18 and above against COVID.

Slimnica Pieejama Razotaja Novavax Eiropa Razota Vakcina Nuvaxovid Stradina Slimnica

Is The Novavax Covid Vaccine Worth The Hype Medpage Today

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl

Fda Advisers Back Authorization Of Novavax Covid Shot Medpage Today

Ecco Nuvaxovid Il Vaccino Di Novavax E Il Quinto Autorizzato Nell Ue Per Il Sars Cov 2 Sif Magazine

European Regulators Warn Over Severe Allergic Reaction After Novavax Shot Bioworld

Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Gov Uk

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary

Coronavaccin Van Novavax Onder Voorwaarden Goedgekeurd Vanaf 18 Jaar Nieuwsbericht College Ter Beoordeling Van Geneesmiddelen

Nuvaxovid Novavax Covid 19 معلومات در باره واکسین Australian Government Department Of Health And Aged Care

Coronavirus Q A On The Nuvaxovid Covid 19 Vaccine Cyprus Mail
Dossier Coronavirus Sars Cov 2 And Covid 19 Suspected Adverse Reactions Reported In The First Three Months Since Start Of Vaccination With Nuvaxovid Paul Ehrlich Institut
Uk Approves 5th Coronavirus Vaccine
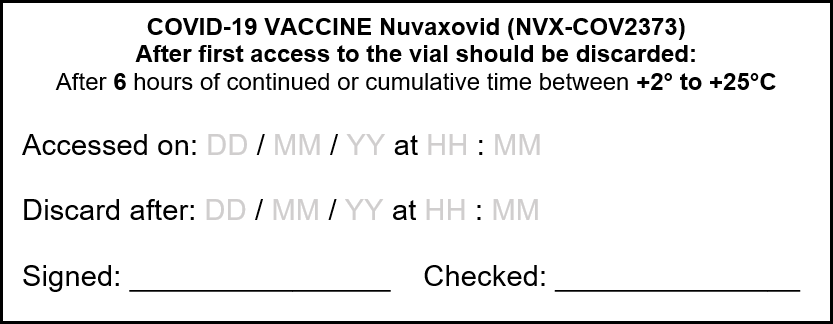
Management Of Covid 19 Vaccine Nuvaxovid Novavax From Refrigerator To Administration Vaccination

European Union Authorizes Novavax Booster

Minzdrav Vakcinoj Novavax Uzhe Mogut Privivatsya Deti S 12 Let Ru Delfi

Middlesex London Health Unit Are You Interested In The Novavax Nuvaxovid Covid 19 Vaccine Mlhu Is Receiving A Limited Supply Of The Vaccine This Spring Call 1 226 289 3560 To Be Added To The Waitlist